How seafood shells could help solve the plastic waste problem
Chemists are finding better ways to extract biodegradable materials from crustaceans and insects, called chitin and chitosan to make bio-based plastic.
Lobster bisque and shrimp cocktail make for scrumptious meals, but at a price. The food industry generates 6 million to 8 million metric tons of crab, shrimp and lobster shell waste every year. Depending on the country, those claws and legs largely get dumped back into the ocean or into landfills.
In many of those same landfills, plastic trash relentlessly accumulates. Humans have produced over 8 billion tons of plastic since mass production began in the 1950s. Only 10 percent of plastic packaging gets recycled successfully. Most of the rest sits in landfills for a very long time (a plastic bottle takes about 450 years to break down), or escapes into the environment, perhaps sickening seabirds that swallow tiny pieces or gathering in the Pacific Ocean’s floating garbage patch (SN Online: 3/22/18).
Some scientists think it’s possible to tackle the two problems at once. Crustaceans’ hardy shells contain chitin, a material that, along with its derivative chitosan, offers many of plastic’s desirable properties and takes only weeks or months to biodegrade, rather than centuries.
The challenge is getting enough pure chitin and chitosan from the shells to make bio-based “plastic” in cost-effective ways. “There’s no blueprint or operating manual for what we’re doing,” says John Keyes, CEO of Mari Signum, a start-up company based just outside of Richmond, Va., that is devising ways to make environmentally friendly chitin. But a flurry of advances in green chemistry is providing some guideposts.
Nature’s scaffold
Chitin is one of the most abundant organic materials in the world, after cellulose, which gives woody plants their structure. In addition to crustaceans, chitin is found in insects, fish scales, mollusks and fungi. Like plastic, chitin is a polymer, a molecular chain made from repeating units. The building block in chitin, N-acetyl-D-glucosamine, is a sugar related to glucose. Chitin and chitosan are antibacterial, nontoxic and used in cosmetics, wound dressings and pool-water treatments, among other applications.
Entrepreneurs are trying to launch new chitin products. Cruz Foam, a company in Santa Cruz, Calif., set out to produce surfboards from chitin, though the company has since pivoted to focus on the much larger market of packaging foam. Polystyrene foam, a common component in both surfboards and food packaging, takes a minimum of 500 years to biodegrade. Company cofounder Marco Rolandi is convinced that his Cruz Foam will biodegrade readily, based on his at-home test. “I put Cruz Foam in my backyard compost and a month later there were worms growing on it,” he says. Eco-friendly surfboards and wound dressings are valuable, but they are niche products — small potatoes that won’t make a dent in the massive amounts of fossil fuel–based plastics. Scientists have proposed large-scale production of chitin or chitosan in the past. But the chemistry for isolating the materials from shell waste has some big drawbacks, so the work didn’t get far.
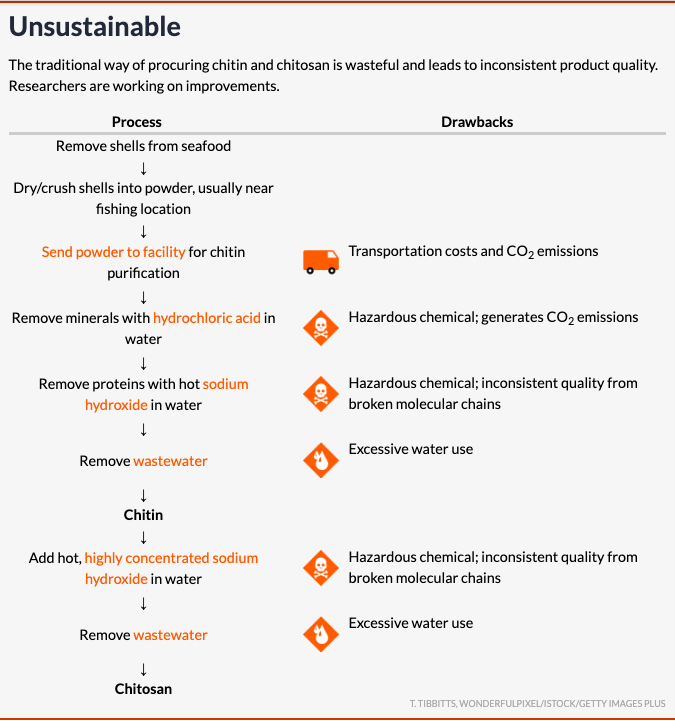
For one thing, pulling out the chitin traditionally requires corrosive chemicals. A crustacean shell contains 15 to 40 percent chitin. To get to the chitin requires removing the protein along with the minerals, largely calcium carbonate, that make the shells stiff. Hydrochloric acid, a strong acid, removes calcium carbonate while generating carbon dioxide emissions; sodium hydroxide, or lye, is a strong base that removes the protein. Producing a single kilogram of chitin requires 10 kilograms of shells, six kilograms of coal for heating purposes, nine kilograms of hydrochloric acid, eight kilograms of sodium hydroxide and 330 kilograms of freshwater. Washing the chitin to remove residual contaminants can use up to an additional 200 kilograms of water.Getting the chitosan requires an extra step: adding hot, concentrated sodium hydroxide solution to the chitin. To do this work in a sustainable way, companies must invest in pricey corrosion-resistant reactors, wastewater treatment and carbon dioxide capture technology.
The harsh reactions used today also sever the long polymer chains that make the materials sturdy, limiting chitin’s and chitosan’s versatility. Mari Signum’s chief technology officer, Julia Shamshina, offers a clothing analogy: It’s impossible to make a sweater with a ball of yarn made only of short threads.
Approaches that reduce or eliminate corrosive reagents, recycle water and keep the polymers strong are in demand, says Pierre-Olivier Morisset of Merinov, a research center in Gaspé, Canada, that helps marine-product companies manage waste and commercialize innovations. “We’re looking for technologies that can produce hundreds of kilograms” of chitin or chitosan with long polymer chains, Morisset says. But developing greener methods is not easy.Seafood suppliers face economic drawbacks as well. Today, U.S. producers pay landfills to take their shells. But those who want to keep the waste out of the landfill and support chitin production must still pay to dry the shells and transport them to often faraway extraction facilities, like Mari Signum. For its part, Mari Signum is changing the equation by paying the transportation bills for its Gulf Coast suppliers. Once Mari Signum is profitable, the company says it will also pay those suppliers for their shells.
When Keyes was a pro bono consultant for an aquaculture business a few years ago, he faced that same food waste decision. The company planned to haul its shells to regional landfills, Keyes says, “until we … tracked down Robin Rogers.”
Get it separated
If the slight drawl doesn’t give Rogers away as a native Alabaman, his ever-present blazer in Crimson Tide red does the trick. A University of Alabama chemist and a cofounder and co-owner of Mari Signum, Rogers started tackling shell waste in 2010, when the Deepwater Horizon oil spill devastated Gulf Coast shrimpers. Mari Signum licensed his technology in 2016. “We found we could take shrimp shell, dissolve the chitin directly and pull it away from everything else,” Rogers says.
The key was to find a liquid that could dissolve chitin, because water cannot. Thousands of repeating sugar units in chitin entangle themselves via countless interactions known as hydrogen bonds, and neither water nor most organic solvents can penetrate that network. Rogers solved that problem by dissolving shell waste in an ionic liquid.
Ionic liquids differ chemically from water and organic solvents. The chemical bonds in a molecule of water — H2O — are covalent: They involve shared electrons. Ionic liquids, in contrast, feature an ionic bond like that seen in table salt, sodium chloride, in which no electrons are shared but the attraction between a positively charged ion, called a cation, and negatively charged ion, or anion, holds things together. While ionic liquids may be saltlike, they are not exactly like table salt. Transforming solid table salt crystals to a liquid requires a temperature about 1.5 times as hot as the surface of Venus, but an ionic liquid can be liquid at room temperature.
Their chemical makeup helps ionic liquids dissolve chitin where other solvents fail. To dissolve chitin in shrimp shells, Mari Signum uses 1-ethyl-3-methylimidazolium acetate. This ionic liquid belongs to the lowest chemical toxicity category, according to United Nations standards. “I tell people half of the ionic liquid we’re using is vinegar,” Rogers says, because negatively charged acetate is present in everyday table vinegar, which is a dilute solution of acetic acid in water. Acetate is a small molecule, in addition to being charged, so it infiltrates chitin’s hydrogen bond networks. Microwave heating activates the hydrogen bonds to enable separation without cutting the polymer chains.
Mari Signum’s process requires neither hydrochloric acid nor sodium hydroxide, and the ionic liquid can be used again and again. The shells’ calcium carbonate is a waste by-product, but the company is looking into providing that waste to makers of paint additives or heartburn medication, where this compound is already used commercially.
Shamshina, Rogers and chemist Paula Berton of the University of Calgary in Canada showed that their chitin, extracted by ionic liquid, can be converted to fibers and hydrogels — materials that can absorb and retain water. In the April 1 ACS Sustainable Chemistry and Engineering, the researchers described potential uses in wound-care materials and biodegradable delivery vehicles for drugs. Plus the group produced novel materials, such as chitin microbeads that could be a biodegradable substitute for the banned plastic microbeads that were used in cosmetics (SN Online: 1/4/19).
Ionic liquids aren’t the only chemistry solution to the chitin extraction problem. Chemist Audrey Moores’ team at McGill University in Montreal published a patent-pending approach to minimizing use of water, or any liquid, online on March 26 in Green Chemistry. Moores’ research assistant Thomas Di Nardo takes shell powder from crustaceans or insects and pounds it with a ceramic ball inside a mechanical mill. This step loosens the hydrogen bonds between chitin’s many chains. Scientists call this approach mechanochemistry.
“Instead of giving energy to your reaction in the form of heating, you’re giving it in the form of mechanical force,” Moores says. Next, instead of employing sodium hydroxide dissolved in water, Di Nardo adds solid sodium hydroxide to the mill, mixes for a few minutes and then transfers the mixture to a steam room–like chamber, where it ages for six days. Each of chitin’s many N-acetyl-D-glucosamine building blocks contains a chemical group that’s akin to a gloved hand. During aging, the sodium hydroxide removes most of the gloves, yielding chitosan.
Compared with the traditional conversion of chitin to chitosan, Moores estimates that the process uses only about a third to a fifth as much energy, an eighth as much sodium hydroxide and a tenth as much water. The process also produces very long polymer chains.
One drawback: Only 10 grams can be made at a time. But Moores received funding from McGill this spring to find ways to produce greater quantities. The next goal is 10 kilograms, and preliminary talks with collaborators suggest it’s doable. Moores is also working with an expert on green plasticizers, to convert her sturdy chitosan into something moldable like the plastics in single-use packaging. A company that makes antibacterial clothing fibers has reached out to her as well, about potentially using her chitosan, which is naturally antibacterial, for the fibers. In May, Moores and Di Nardo launched a spin-off corporation, ChitoDry.